Neuroinflammation causes life-altering symptoms
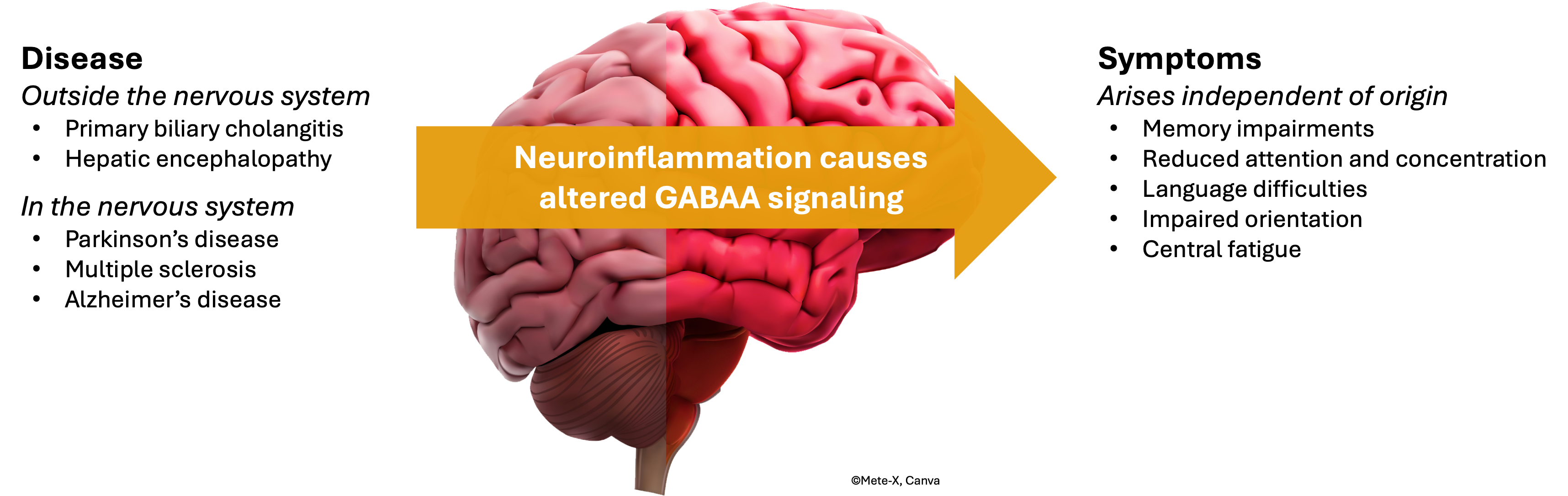
Chronic neuroinflammation – persistent low grade inflammation in the brain – may arise in a range of diseases and is linked to symptoms like memory loss, impaired concentration, and severe fatigue. These cognitive and physical challenges significantly impact patients’ daily lives and represent a major unmet medical need. Neuroinflammation is also a key feature in the progression of neurodegenerative disorders such as Parkinson’s disease and Alzheimer’s disease.
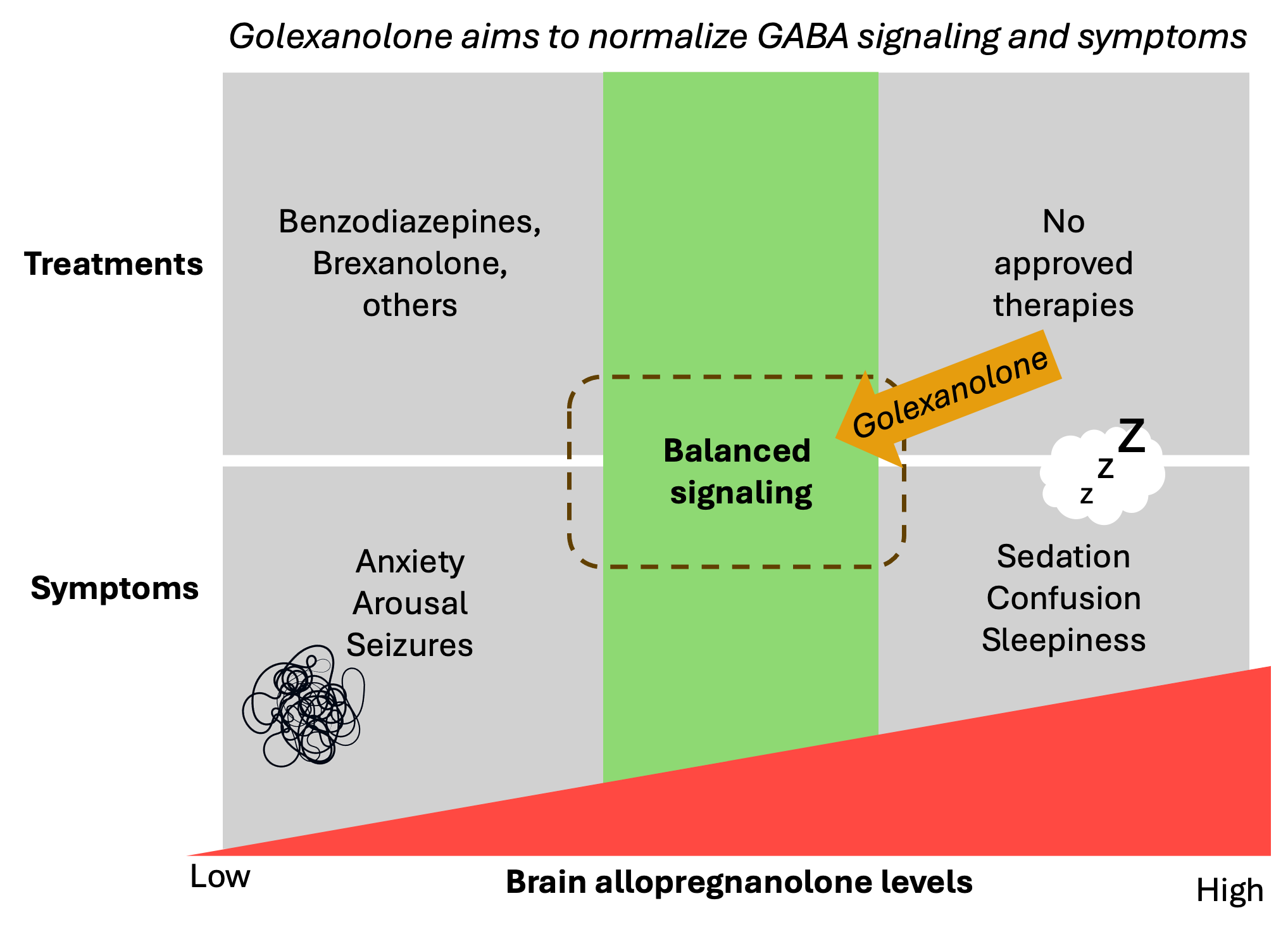
A key driver of these symptoms is overactivation of the GABAA receptor, which disrupts the brain’s balance between activity and rest. Traditional approaches to correcting this imbalance by blocking the receptor have resulted in improved neuroinflammation and neurotransmission but with unacceptable side effects, highlighting the need for safer, more targeted therapies.
A new class of drugs addressing cognitive symptoms
Umecrine Cognition’s research has identified that neuroinflammation raises levels of allopregnanolone, a hormone in the brain that increases GABAA receptor sensitivity, contributing to debilitating symptoms. The company’s lead candidate, golexanolone, is a first-in-class therapy designed to modulate the GABAA receptor in a controlled manner, restoring neural balance and removing neuroinflammation without the risks observed in other treatments.
Preclinical studies show that golexanolone normalizes brain signaling, reduces harmful immune activation, and alleviates fatigue, cognitive impairment, and motor dysfunction in models of primary biliary cholangitis (PBC), Parkinson’s disease, and hepatic encephalopathy. Golexanolone has previously been evaluated in a Phase 2 clinical study in HE and is currently being evaluated as a potential treatment for cognitive impairment and fatigue in a Phase 1b/2a study in PBC patients.
Umecrine Cognition thereby aims to offer a novel, mechanism-based approach addressing a high-value segment of the CNS therapeutics market, with significant potential for clinical and commercial impact.